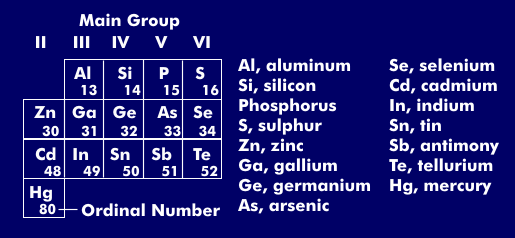
III-V compound semiconductor
The designation III-V compound semiconductors goes back to the group of the periodic table from which the compound semiconductors originate. The main group III of the periodic table includes boron (B), aluminum (Al), gallium ( Ga) and indium( In), the main group V phosphorus (P), nitrogen (N), arsenic ( As) and antimony (Sb).
III-V compound semiconductors include gallium arsenide( GaAs), gallium nitride( GaN), indium gallium nitride( InGaN) and indium phosphide( InP). These compound semiconductors have much shorter switching speeds compared to conventional silicon( Si) and can operate at higher voltages. They can therefore be used in microelectronics and microwave technology up to 100 GHz.
Gallium arsenide (GaAs) is used to make integrated circuits for microwave technology, infrared LEDs, Gunn diodes, and optical integrated circuits( OIC) for integrated optics. Other semiconductor compounds are used in optronics, photonics and photovoltaics. For example, in photoelectric sensors, optical transmitters and receivers, solar modules, and various other components.
In addition to the III-V compound semiconductors, there are also the IV-IV compound semiconductors, which are primarily based on silicon and germanium.