fiber optic cable
A fiberopticcable consists of one or more optical fibers housed in and protected by a common cable sheath. They are available for a wide variety of applications and designs, differing in the number of optical fibers.
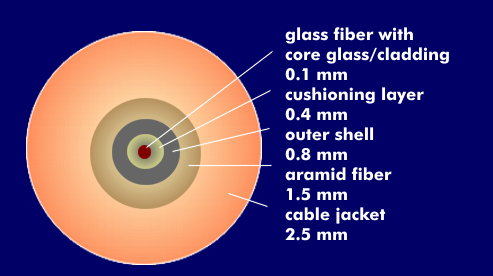
Fiber optic cables consist of a cable jacket with armor that can contain up to 144 optical fibers. The cable sheath and cable construction protect the optical fibers from mechanical stresses such as tensile loads and compressive stresses, moisture and corrosion. In addition, the cable jacket must be flexible, it must withstand high tensile loads during cable installation, and it must be flame retardant or flame retardant. The core glass of the optical fiber is protected by a cladding glass, the primary coating and the secondary coating.
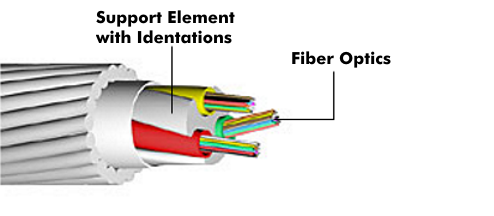
To improve strength and provide strain relief, the secondary coating is wrapped with aramid yarn. In various LwL cables, additional support elements are installed to absorb compressive forces. In the case of chamber cables, the optical fibers are inserted in helical grooves and are thus protected against mechanical stresses. In the case of unfavorable cable design or improper installation, the fiber can be subjected to high stresses and its damping behavior can change.
The transmission behavior of FO cables is determined solely by the optical fibers and the transmission of the modes. In order to maintain the advantage of potential separation between data source and receiver in the cable, the installation of metal elements in FO cables is avoided whenever possible. This is referred to as metal-free FO cables.
FO cables are primarily used in the areas of patch cables, breakout cables and installation cables. While patch cables have one or two optical fibers, breakout cables are FO cables with up to 48 optical fibers, and outdoor cables have loose tubes with up to 144 optical fibers. As far as cable classifications are concerned, all the aforementioned fiber optic cables can meet OM classes OM1 to OM4, and the single mode fibers can meet OS classes OS1 and OS2.