molton carbonat fuel cell (MCFC)
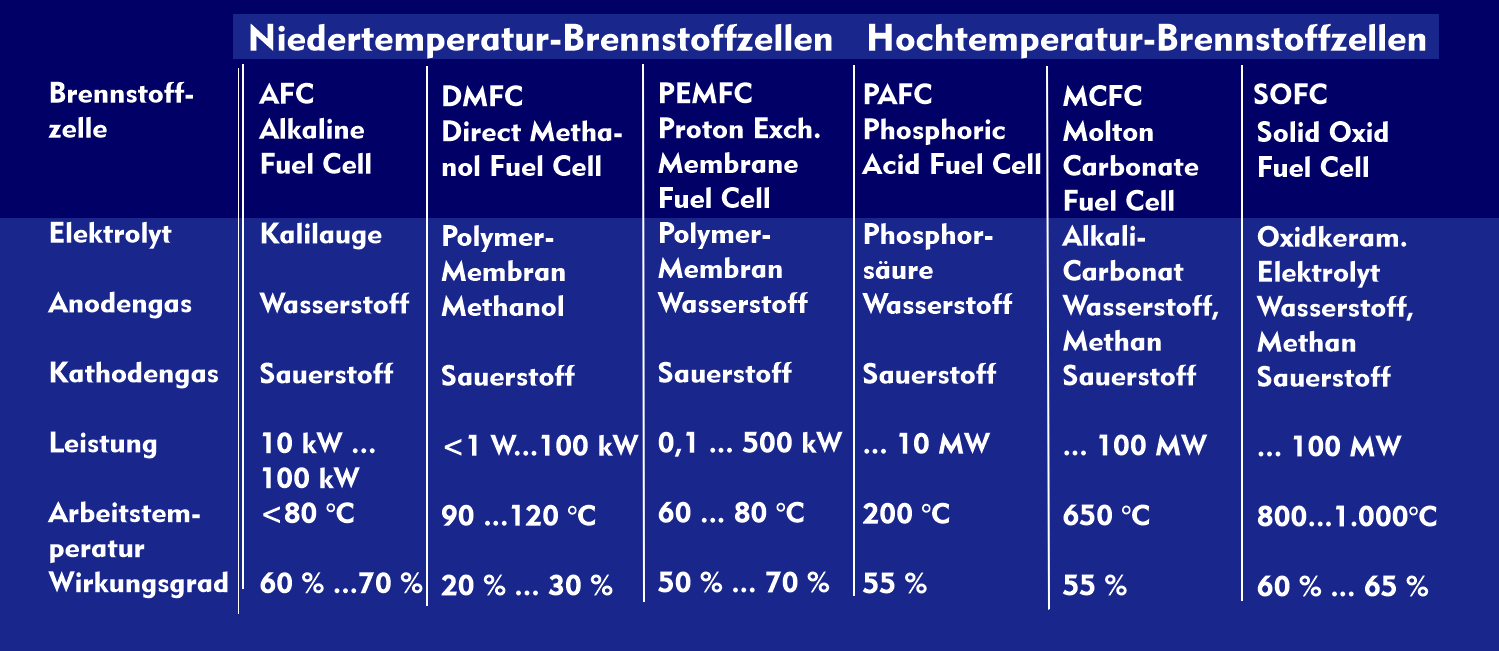
Molten carbonate fuel cells (MCFCs) take their name from the electrolyte used, which consists of molten carbonate salts of lithium carbonate and potassium carbonate. With an operating temperature of 650 °C, MCFC fuel cells belong to the high-temperature fuel cells.
In molten carbonate fuel cells, carbon dioxide and oxygen are fed into the fuel cell, where they react to form carbonate ions. The carbonate ions act freely in the molten salt electrolyte. MCFC fuel cells used hydrogen, methane and carbon monoxide as fuel gas, which is obtained from natural gas and biogas and fed to the anode side. The positively charged hydrogen ions migrate through the electrolyte from the anode to the cathode. The anode consists of a porous nickel alloy. The electrons generated at the anode travel through an external circuit, which supplies current along the way, and return to the cathode.
The efficiency of MCFC fuel cells is up to 60 %, which is higher than that of various other fuel cells. They are used in combined heat and power plants and large power plants with outputs of up to one hundred megawatts.